Gingival health benefits of essential-oil and cetylpyridinium chloride mouthrinses: A 6-month randomized clinical study44
- Cortelli SC, Cortelli JR, Shang H, Costa R, Charles CA. Am J Dent. 2014;Vol.27, No.3.
Objective
This randomized, single center, examiner-blind, controlled, parallel-group, 6-month clinical study compared the antiplaque/antigingivitis potential of an essential oil (EO) versus a 0.07% cetylpyridinium chloride (CPC)-containing mouthrinse. A 5% hydroalcohol solution was included as a control group.
Methodology
354 healthy volunteers (18-71 years of age) were enrolled in this clinical trial; 338 subjects completed the study. At baseline, 1-, 3-, and 6-month visits, subjects received an oral examination, gingivitis (MGI), gingival bleeding (BI) and plaque assessments (PI). Following randomization, subjects received a prophylaxis and began brushing twice daily with the provided fluoride toothpaste and rinsing twice daily with 20 mL of the assigned mouthrinse for 30 seconds.
Results
When compared to CPC, EO was statistically significantly superior at all post-baseline time-points. EO showed increasing reductions in MGI of 10.5%, 20.3% and 30.7% as well as reductions in PI of 12.7%, 23.7% and 32.6% at 1, 3 and 6 months, respectively. When analyzing the number of healthy sites (MGI scores of 0 or 1), the beneficial effect of the EO-containing mouthrinse is 45.8% greater than using a CPC-containing mouthrinse and 59.8% greater than placebo.
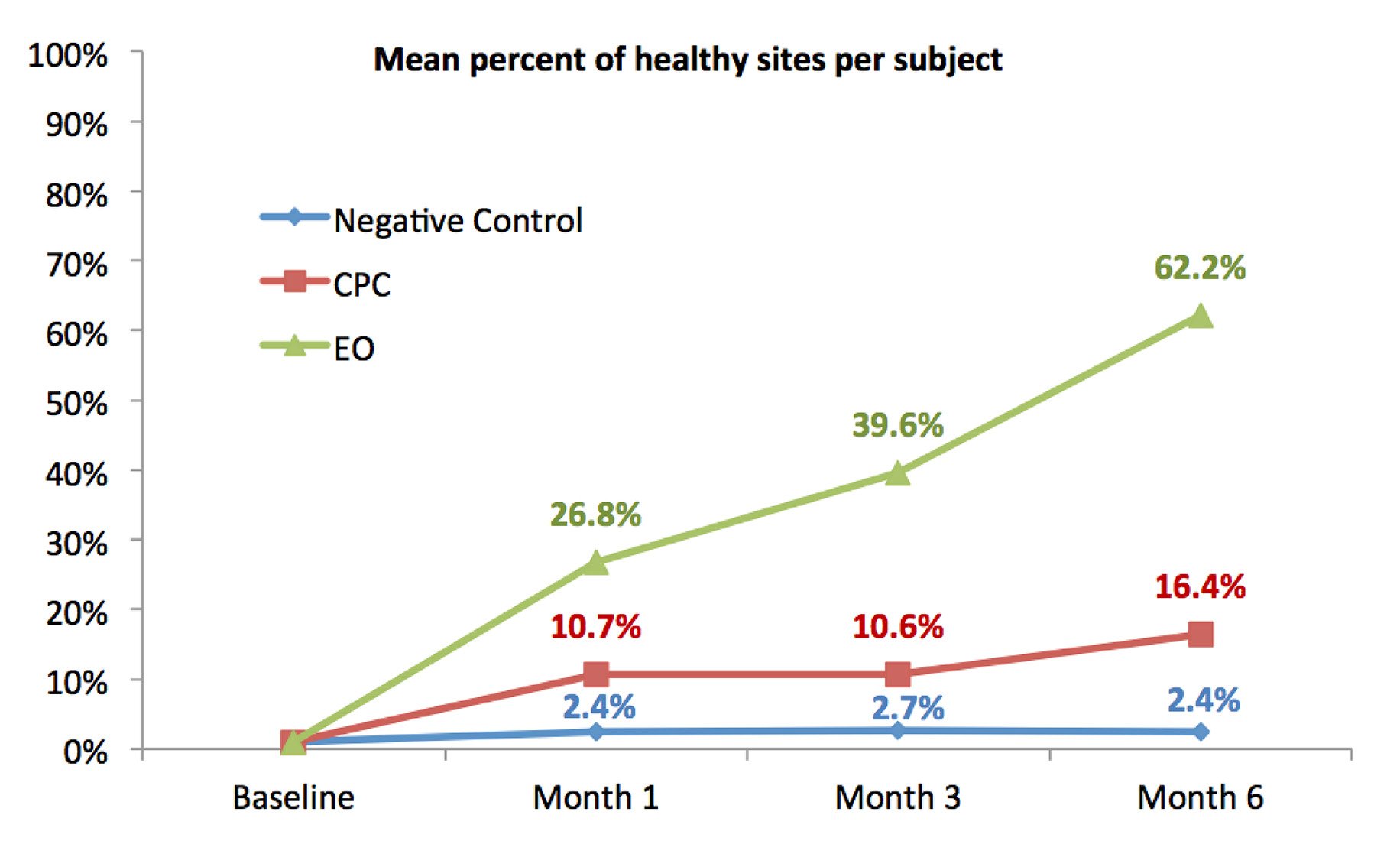
Fig.4. Mean percent of healthy sites per subject.
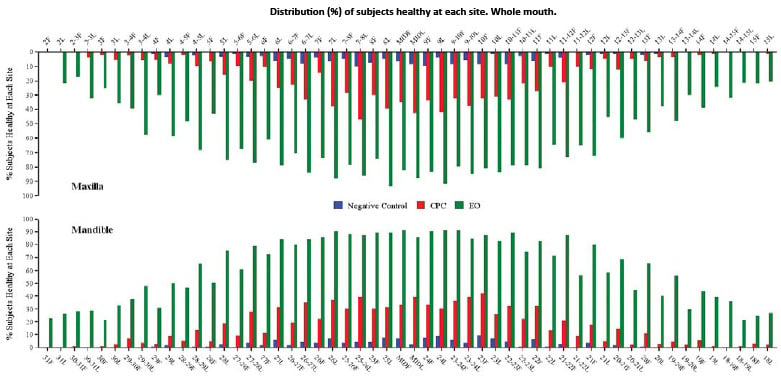
Fig. 2. Distribution (%) of subjects healthy at each site. Whole mouth.
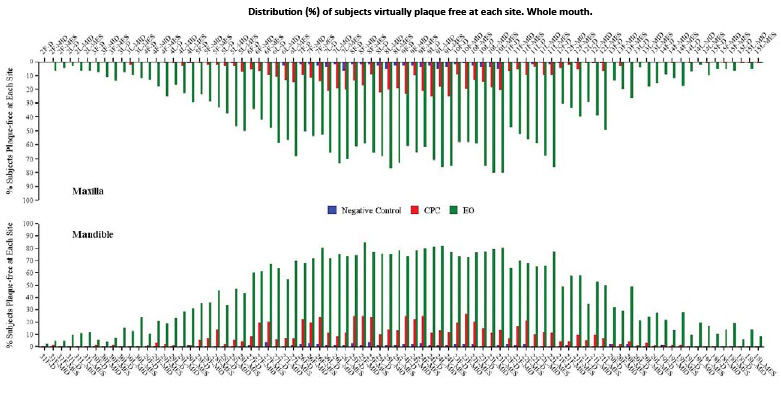
Fig. 3. Distribution (%) of subjects virtually plaque free at each site. Whole mouth.
Conclusion
The results of this study demonstrated the clinical superiority of a mouthrinse containing EO versus 0.07% CPC in the long-term management of plaque and gingivitis.
LISTERINE® Antiseptic
Explore our wide selection of LISTERINE® Antiseptic products