Comparative effects of 2 chemotherapeutic mouthrinses on the development of supragingival dental plaque and gingivitis41, 42
- Overholser CD, Meiller TF, DePaola LG, Minah GE, Niehaus C. J Clin Periodontol. 1990;17:575-579
Objective
Determine the efficacy of 2 mouthrinses — LISTERINE® Antiseptic and Peridex® — used as supplements to regular oral hygiene measures in reducing supragingival dental plaque and gingivitis (N=124).
Methodology
Double-blind, controlled, parallel design clinical study of healthy male and female subjects (aged ≥21 years) with preexisting plaque and gingivitis, but without evidence of periodontitis.
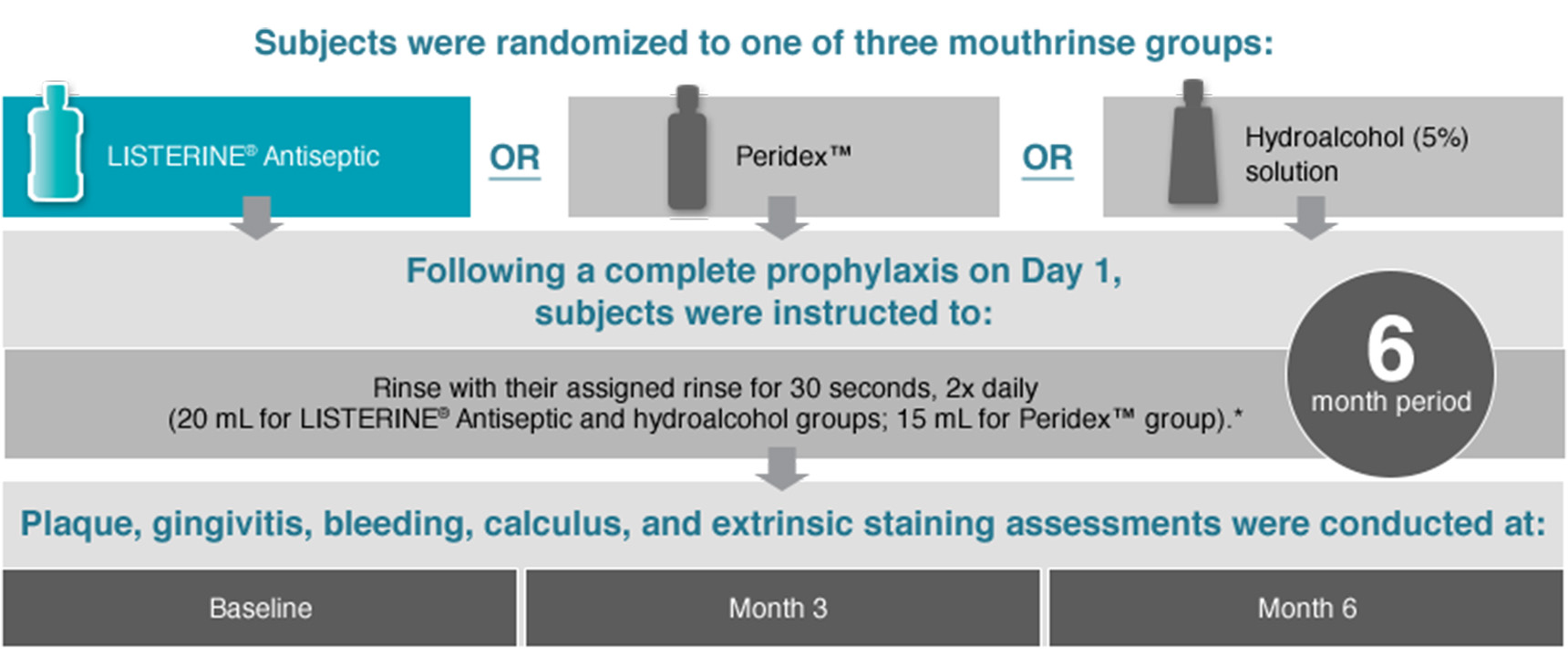
Subjects were randomized to one of three mouthrinse groups:
- LISTERINE® Antiseptic
- OR
- PeridexTM
- OR
- HYdroalcohol (5%) solution
Following a complete prophylaxis on Day 1, subjects were instruted to:
Rinse with their assigned rinse for 30 seconds, 2x daily (20 mL for LISTERINE® Antiseptic and hydroalcohol groups; 15 mL for PeridexTM group)*. 6 month period
Plaque, gingivitis, bleeding, calculus, and extrinsic staining assessments were conducted at:
- Baseline
- Month 3
- Month 6
Results
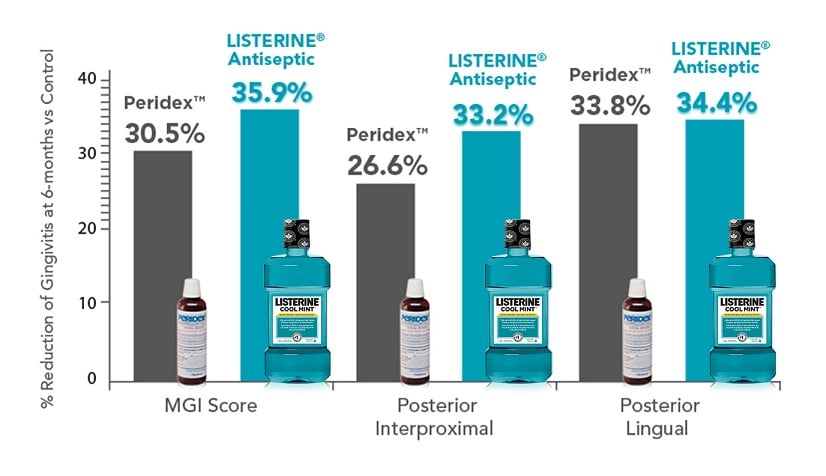
At 6 months, gingivitis development was inhibited by 35.9% in the LISTERINE® Antiseptic group and by 30.5% in the Peridex™ group, when compared to the hydroalcohol control (P<0.001).
At 6 months, gingivitis development—in posterior regions—was inhibited by 33.2% and 34.4% in the LISTERINE® Antiseptic group and by 26.6% and 33.8% in the Peridex™ group, when compared to the hydroalcohol control (P<0.001).42
Conclusion
LISTERINE® Antiseptic produces gingivitis reductions comparable to Peridex™ at 6 months (including gingivitis in posterior regions).
*During the study, subjects followed their usual oral hygiene and dietary habits.
LISTERINE® Antiseptic
Explore our wide selection of LISTERINE® Antiseptic products