A comparison of the effects of 2 commercially available nonprescription h3 flow rates and xerostomia24
- Kerr AR, Katz RW, Ship JA. Quintessence Int. 2007;38(705):41-48.
Objective
To determine if 2 commercial mouthrinses, 1 alcohol-based (LISTERINE COOL MINT®) and 1 non-alcohol-based (Act® Anticavity Fluoride Mouthwash — Alcohol Free, Mint), affect salivary flow and symptoms of dry mouth in nonxerostomic adults (N=20).
Methodology
Observer-blinded, randomized, crossover study of nonxerostomic subjects (aged 18-85 years).

Subjects were randomized to one of two mouthrinse groups:
- Alcohol-containing (LISTERINE® Antiseptic)
- OR
- Nonalcohol containing (Act® Mint).
Subjects rinsed with 20 mL of their assigned rinse for 30 seconds at the first study visit, and then continued using it twice daily for one week at home
At second study visit, subjects rinsed with their assigned rinse, waited 90 minutes (washout period), and were then crossed over to the other rinse group.
At crossover, subjects rinsed with 20 mL of the other rinse for 30 seconds in the clinic, and then continued usin it 2x daily for one week at home.
At the third and final study visit, subjects rinsed one last time with their crossover rinse.
At all 3 study visits investigators assessed salivary flow rates and perceived mouth dryness.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Less than 50% on a 100-mm dry mouth VAS (Visual Analog Scale) questionnaire | Presence or history of oral cancer |
| More than 0.1 mL/min for unstimulated whole saliva | More than 50% on a 100-mm dry mouth VAS questionnaire |
| Less than 0.1 mL/min for unstimulated whole saliva |
Results
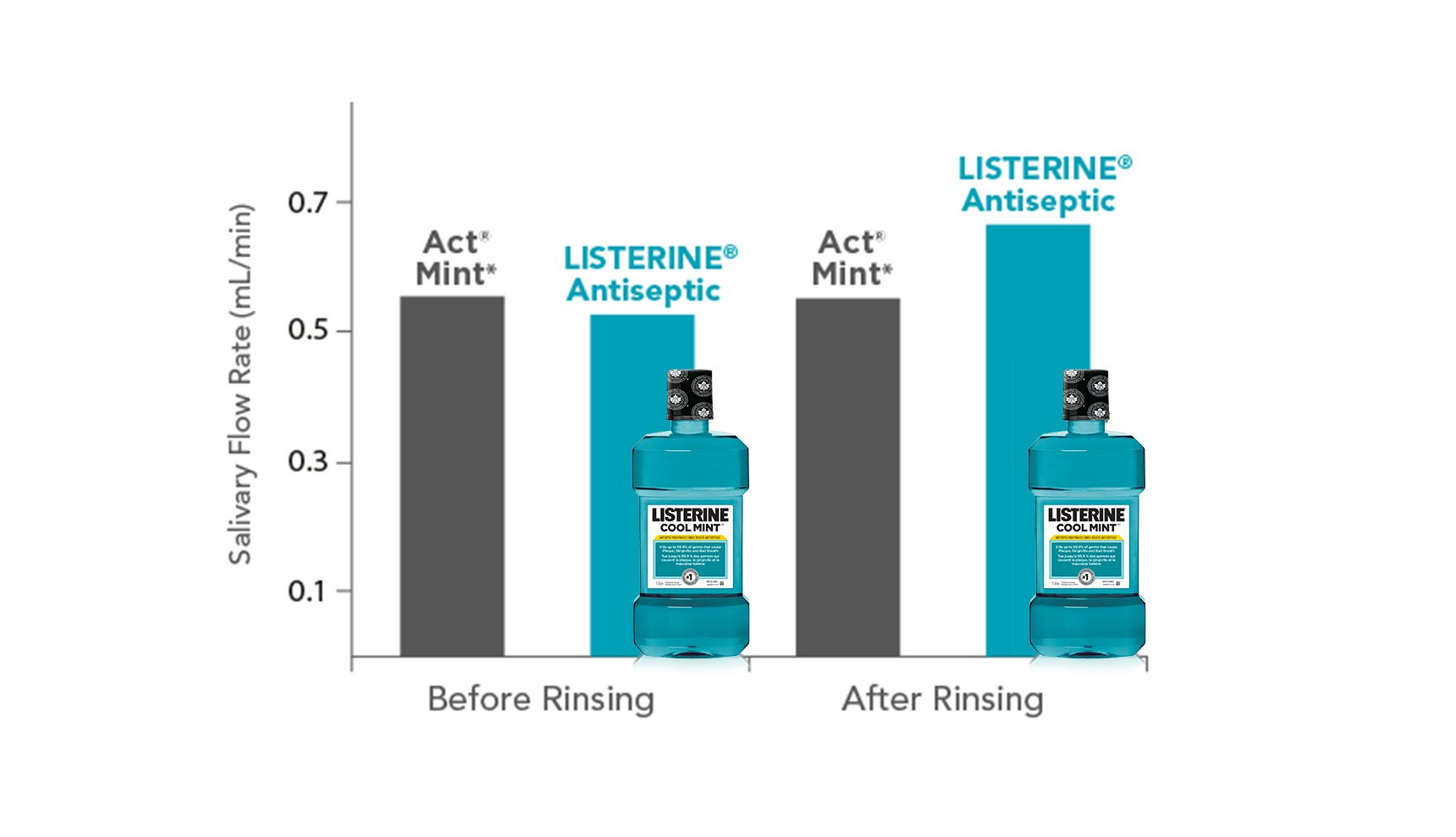
After 1 week of mouthrinse use, combined data (before and after crossover) revealed no significant differences in flow rates between groups (P >0.05).
Conclusion
There were no differences in salivary flow rate and sensation of mouth dryness between alcohol- and non-alcohol-containing mouthrinses.
*Non-alcohol based.
Safety & Tolerability
Find out more about LISTERINE® Brand products’ safety and tolerability.